|
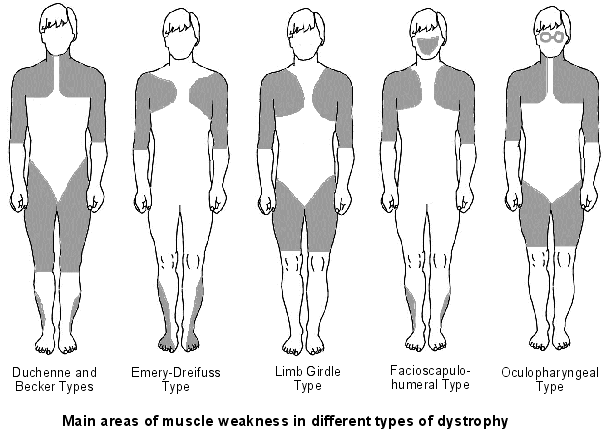
The molecular basis of DMD was first determined in the 1980s. DMD symptoms usually begin to show between the ages of 3-5 and affect 1 in 3,500 patients. At the age of 20 patients are usually unable to walk so bound to a wheelchair. In 1986 Gowers observed that familial cases were more common than sporadic cases. P.E Becker discovered that there is a milder form of DMD where symptoms usually show after the age of 12 and affects 3 per 100,000 new born. He also discovered that X-linked disorders have slower progression. It does not affect reading frame but has DMD gene deletions. The protein involved in DMD is dystrophin. The dystrophin gene is the largest gene that encompasses 206 million base pairs and contains 79 exons. It is the main proteins that link the cytoskeleton to the extracellular matrix. DMD has shown variation in muscle size and penetration of connective tissue. Patients suffering from DMD have a deletion of 45 exons and in BMD 35 exons. Deletions have been found on Xp21.
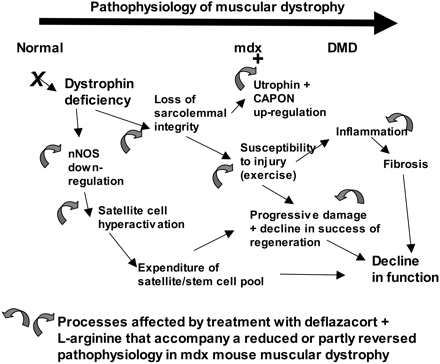
The cloning of the dystrophin gene in the 1980s led to the discovery of physiological consequences of DMD. There are three major proteins that were first discovered known as dystrophin associated protein complex (DAPC). Dystrophin is a rod shaped cytoplasmic proteins that links actin fibres in cortex of muscle cells to extracellular basal lamina (connective tissue) by forming a bridge between actin and transmembrane protein complex called (dystrophin-associated sarcoglycan complex) (DASC) located in sarcosplasma. Without dystrophin an improper complex is formed. DMD gene has 79 exons with promoter regions scatters across 2.4 Mb. The first of the proteins is known as dystroglycan. The dystroglycan complex is a membrane-spanning complex composed of two subunits, alpha- and beta-dystroglycan. Alpha-dystroglycan is a cell surface peripheral membrane protein which binds to the extracellular matrix (ECM), whereas beta-dystroglycan is an integral membrane protein which anchors alpha-dystroglycan to the cell membrane. The dystroglycan complex provides a tight link between the ECM and cell membrane. No patients have shown the mutation is the studies of Dalkillic et al (2003). The second proteins are Dystrobrevins and Syntrophins. These have been found to bind to dystrophin; these include multiple iso-forms of alpha and beta dystrobrevin and three syntrophin iso-forms. Alpha dystrobrevin is expressed in the skeletal muscle, and localized in the sarcelloma, and interacts with dystrophin. No mutations have been found but mice models with this mutation shown mild dystrophy. Three iso-forms of syntrophin have been expressed in the skeletal muscle and shown to interact with dystrophin and dystrobrevin, and bring (nNOS) nitric neurol oxide synthesis. nNOS cause vasodilation near the muscle tissue in order for blood flow to occur for contraction. Less syntrophin would mean less nNOS. No patients have shown to have this except studies of mice. Finally the last protein is the sarcoglycan-sarcospan complex. These forms of sarcoglycan interact with each other strongly that give rise to MD. DMD patients have shown to decrease in this protein and lost at the membrane. Other types of proteins that are involved are caveolac, and there is an abnormal size and number. This is found in all muscle types and interacts with C-terminal. DMD is an X-linked recessive disorder as the mutated gene is found on the X chromosome. The X chromosome is important for the development & growth. Females can be carriers of this disease if one X chromosome is affected as the other X chromosome would compensate. As for males they can either be normal with without the affected gene or affected. A female carrier can pass her affected X chromosome to son/daughter, therefore the son has a 50% of being infected and the daughter has 50% of being a carrier. There are strategies used to identify and isolate genes involved in this monogenic disorder. 1) Cytogenetic rearrangement results in DMD having location sub-chromosomal location to Xp21. Xp21 fusion with 285 ribosomal RNA nucleus on chromosome 21, allowing marker XJ1.1 à closely linked to be isolated. 2) Use of DNA from a boy called ‘BB’ who suffered from 3 x-linked disorders: DMD, chronic granulomatous disease, retinitis pigmentosa. Genes responsible for these disorders are closely linked. Appeared that BB’s X chromosome cytogentically carried a visible deletion that affected part of all 3 genes. DNA by this deletion isolated from XXXXY cell line using substractive hybridization techniques. One of these clones, pERT87 closely linked to DMD mutation sites. pERT87 and XJ1.1 clones used to map locus constructing long-range restriction map. pERT used to probe cDNA libraries resulting in isolation of cDNA clones together spanned 14-kb mRNA of locus.
3 Comments
Hafsa Abbas
12/24/2020 01:33:40 am
Thank you
Reply
Leave a Reply. |
A GOOD HEALTH MAKES YOU RICHHealth is crucial in every single person’s life. Its something that money can’t buy. Thus a good health makes you rich so look after it. Archives
May 2017
Categories |
 RSS Feed
RSS Feed