|
Herpes Simplex Virus
Herpes Simplex Virus (HSV) belongs to the alphaherpesvirus subfamily of the herpesvirus. Herpesvirus is a linear ds DNA enveloped virus with viral glycoprotein spikes with a genome of 150kb. HSV share some of its antigens with other herpesviruses, in particular Varicella Zoster Virus (VZV). HSV can be classified into 2 subtypes based on serology between genomes HSV 1 and HSV 2 which share 50-70% homology. Both are ubiquitous and contagious HSV is spread by contact (close/sexual/maternal) as the virus is shed in saliva, tears, genital and other secretions. The primary infection is usually trivial and subclinical in most individuals, 2 peaks of primary infection usually found those younger than 5 years of age or when sexually active. Around 10% of population acquires HSV infection through the genital route. Generally HSV 1 causes infection in the facial area, HSV 2 causes mainly genital herpes. Following primary infection, 45% of orally infected and 60% of patients with genital herpes will experience recurrences. During primary infection, HSV spreads locally and viraemia is rare which has localised infection at site of entry and then the virus spreads down neurones where it becomes latent in the craniospinal ganglia. There’s three distinct stages involved which are establishment, maintenance and reactivation. The virus establishes latency in the cransiospinal ganglia. The exact mechanism of latency in not known but this is where HSV is able to escape the immune response and persist indefinitely in the latent state. The mechanism may be either a) True latency – virus is non-replicative and maintained within the cell by integration into the cellular chromosome or in an episomal form. b) Virus persistence – can be described as dynamic latency whereby there is a tightly controlled low grade productive virus infection not leading to the lysis of the cell. During latent infection, HSV express Latency Associated Transcript (LAT) RNA. LAT is known to regulate host cell genome and interferes with apoptosis. By maintaining the host cells, LAT expression preserves the virus and outbreaks of non-latency occurs. Reoccurency can be asymptomatic/symptomatic, viral shedding occurs to produce further infection. Herpesvirus DNA contains a gene protein called ICP4, which is an important transactivator of genes associated with HSV 1. ICP4 binds to a protein known as human neuronal restrictive silencing factor. This prevents the initiation of transcription from the gene and other viral genes involved in the lytic cycle. Another HSV protein reverses the inhibition of ICP4 protein synthesis. ICP0 dissociates (NRSF) from ICP4 and prevents silencing of viral DNA. Which is reactivated which triggers off reoccurances, this can be by physical, psychological stress, infection (pneumococcal, meningococcal), fever, irradiation (sunlight), menstruation etc. the usual site for implantation is skin or the mucous membrane. HSV is involved in a variety of clinical manisfestations which include: 1) Acute gingivostomatitis – most common in herpetic infection, pain and bleeding in gums, ulcers, enlarged neck glands, lasts 13 days. 2) Herpes labialis (cold sore) – 45% experience reactivation 3) HSV genitalis – can be primary, recurrent or initial, lesions are prone to secondary bacterial infection e.g. S.aureus, streptococcus, trichomonas and candida albicans. Dysuria is the common compliant in severe cases, may be urinary retention. 4) Ocular Herpes – lesions in the external eye to the inner eye 5) HS encephalitis (HSE) – most complicated HSV. There are 2 forms – neonatal and focal. Neonatal where the brain is almost liquefied. Focal is where the temporal lobe is mostly affected. Without treatment mortality rate is 70%. In all general practice IV acyclovir is given in all cases for suspected HSE until results are available. 6) Neonatal herpes – baby is infected perinatally during passage through the birth canal. Premature rupturing of both membranes are a recognised risk factor. It disseminates when there is a florid primary infection in the mother. There is a smaller risk from recurrent lesions in the mother, probably due to the lower viral load and the presence of specific antibody. 7) Herpetic whitlow – arises from the implantation of the virus into the skin and affecting fingers. 8) Eczema Herpeticum – occurs in patients who have eczema. 9) Meningitis – milder than bacterial infection. Neonatal herpes may be caused by HSV1 or 2 which either type may be caused by genital herpes. Almost all cases occur due to direct contact with infected maternal secretions although some cases are due to postnatal transmission. Risks become greater in the third trimester of pregnancy, within 6weeks of delivery as viral shedding may persist and the baby is likely to be born before the development of protective maternal antibodies. Therefore viral cultures are taken every week during the last 6 weeks of pregnancy to detect any recurrent herpes episodes, positive cultures near labour would undergo caesarean section. Data from USA suggest around 2% of women acquire genital HSV infection in pregnancy, most of these are asymptomatic or unrecognised. Its difficult to distinguish clinically between recurrent and primary genital HSV as many first HSV infections are not try primary infections. Disseminated herpes is commonly reported in immunocompromised pregnant women, as those infected with HIV. Both HSV and HIV results in an increased replication of both viruses, genital reactivation of HSV may increase the risk of perinatal transmission of both HIV and HSV. Symptomatic genital herpes is confirmed by direct detection of HSV. Specimens from ulcerated lesions are sampled by swabbing the base of the ulcer and vesicular lesions are de-roofed and fluid is swabbed, this is analysed by PCR. Although HSV seriological testing (IgG antibodies to HSV 1 and 2) is now widely available, but the management of herpes in pregnancy has not yet been fully understood. Pregnant women with genital herpes should be put onto IV acyclovir as it reduces the duration and severity of symptoms which decreases the duration of viral shedding. Acyclovir is well tolerated in pregnancy so dose adjustment is not necessary. The presence of antibodies of the same type of HSV isolated from genital swabs would confirm the episode to be recurrent rather than a primary infection. The rationale for elective caesarean section for the prevention of neonatal herpes is implanted to reduce exposure of the foetus to HSV in genital secretions. However an observational study in USA indicated that caesarean section was not protective against neonatal herpes when the membranes had been ruptured for more than 4 hours. The membranes should be left intact for as long as possible. invasive procedures such as foetal scalp electrode monitoring and foetal blood sampling may also associate with neonatal transmission. The neonate will then need to consider treatment with IV acyclovir. Another study showed 46 pregnant women whose first episode of genital herpes showed in pregnancy and at 36 weeks they received a daily dose of acyclovir or placebo until delivery. The protocol permitted vaginal delivery only if there were no HSV lesions at the time of delivery. No child developed neonatal herpes with either groups. Accurate way of determing the risk of acquiring HSV infection in pregnancy is by a sensitive type-specific serological tests which are commercially available and it determines a woman’s susceptibility to HSV infection in pregnancy. This should be taken either in early pregnancy or in the third trimester. And HSV-seropositive woman can be reassured that her risk of transmission to the neonate is very low. An HSV-seronegative woman is susceptible to genital herpes, in which case they can reduce the risks further by acyclovir treatment. HSV diagnosis is by: 1) Light microscopy – cells from the base of the lesion or mucous surface may reveal intranuclear inclusion (Lipschutz inclusion bodies). Infected cells may show ballooning and fusion. 2) Electron microscopy – its not a sensitive tool for the detection of HSV, except in the case of vesicle fluids containing 108 per millilitre. However microscopy cannot distinguish between different herpes viruses. 3) Direct examination by antigen detection – specimens are treated in ice-cold acetone. FITC is generally used for staining, its 90% sensitive and 90% specific than light and electron microscopy, however expertise is more demanding. PCR from swabs is used routinely for the diagnosis of HSE. 4) Virus culture – HSV 1 and 2 are easier to cultivate, fresher the lesion the better chance of recovery. Inoculation carried out asap. A typical cytopathic effect can be seen after day 1. Other viruses can mimic CPE of HSV, identification can be carried out by immunofluorescence. 5) Serology – complement fixation tests (CFTs) and indirect haemagglutination (IFT). Weak antigenic cross reaction with VZV causes problems in these tests. So ELIZAs and RIAs are gradually replacing them. Treatment of HSV is usually via the administration of Acyclovir which selectively inhibits HSV DNA polymerase and causes premature chain termination when it competes with the guanine triphosphate for the newly synthesised viral DNA. Varicella Zoster Virus VZV is a dsDNA enveloped virus which has only one antigenic serotype although there is some cross reaction with HSV. Primary varicella is an endemic disease and is one of the classic diseases of childhood, mostly prevalent at 4-10years of age. Most people become infected before adulthood but 10% of young adults remain susceptible and it occurs sporadically and evenly throughout the year and peaks in sring. The virus gains entry via the respiratory tract and spreads shortly after to the lymphoid system. After an incubation period of 14days, the virus arrives as its main target organ, the skin! Following primary infection, the virus remains latent in the cerebral or posterior root ganglia. In 10-20% of individuals, a single recurrent infection occurs after several decades. The virus reactivates in the ganglion and tracks down the sensory nerve to the area of the skin innervated by the nerve, producing a varicella form rash in the distribution of a nerve dermatome. Congenital VZV occurs in women which the primary infection is rare during pregnancy. In the first 20 weeks of pregnancy, there is up to 3% chance of transmission to the foetus which leads to scarring of the skin, hypoplasia of limbs, CNS and eye defects, death in infancy. Neonatal VZV can cross the placenta in the late stages of pregnancy to infect the foetus congenitally. Neonatal varicella may vary from a mild disease to a fatal disseminated infection. If a rash in the mother occurs more than 1 week before delivery then sufficient immunity would have been transferred to the foetus. Zoster immunoglobulin should be given to the susceptible pregnant woman and also given to infants whose mothers develop VZV during the last 7 days of pregnancy or the first 14 days after delivery. Clinical presentations of VZV are so characteristic that laboratory confirmation is rarely required, it is usually required for atypical presentations in the immunocompromised. Skin legions progress rapidly through the stages of macules to papules to vesicles which rapidly breakdown crust formation. Patients with VZV are considered to be infectious 2 days before the appearance of rash, and 7 days after onset when vesicles have crusted. Diagnosis same as HSV- Immunofluorescence on skin scrapings can distinguish between the two. Serology – presence of VZV IgG is indicative of past infection and immunity. The presence of IgM is indicative of recent primary infection. Acyclovir is given promptly to immunocompromised individuals with varicella infection. The Internation Herpes Management Forum recommends that antiviral therapy should be offered routinely to all patients over the age of 50 with VZV. Preventative measures should be considered for individuals at risk of contracting severe varicella infection, so a passive immunisation should be given. Zoster immunoglobulin (ZIG) is the preparation of choice but its very expensive, where ZIG is not available HNIG should be given instead. Also a live attenuated vaccine is available however the virus may become latent and reactivate later on in immunocompromised individuals. Cytomegalovirus (CMV) CMV belonds to beta herpesvirus subfamily and is a dsDNA enveloped virus. The structure of the genome for CMV is similar to other herpesviruses, however it has a total of 4 different isomers. CMV is one of the most successful human pathogens that be transmitted vertically or horizontally with little effect on the host. Transmission may occur in utero, prenatally or postnatally (saliva but sometimes can be sexually transmitted through blood). Once infected the person carries the virus for life which may be activated from time to time which infectious virions appear in the urine and saliva. Reactivation can lead to vertical transmission in people who have experienced primary infection to be reinfected with another or same strain of CMV. In developed countries 40% of adolescents are infected and ultimately 70% of the population is infected. In developing countries over 90% of people are infected. Clinical manisfestations of congenital infection may result in cytomegalic inclusion disease. CMV is not the second common cause of mental retardation after Down’s Syndrome. Perinatal and postnatal infections are usually asymptomatic. Immunocompromised patients such as transplant recipients and AIDS patients are prone to severe CMV in which case reactivation is symptomatic. Diagnosis of CMV same as HSV 1) Direct detection - PCR for CMV-DNA can be used in some centres however there are problems with interpretation (how its diagnosed now) 2) Viral isolation – useful is DEAFF which provides a result in 24-48 hours as the fluorescent detects early antigens. Treatment: Congenital infections – mother is usually told about the chances of her baby having cytomegalic inclusion disease and its complications. She can decide whether or not she wants to keep the baby. Unlike rubella congental infection with CMV is not trimester related. Perinatal and postnatal infection – not necessary to treat patients. Immunocompromised patients – necessary to make early diagnosis of CMV infection to give prompt antiviral therapy such as ganciclovir, forscarnet and cidofovir. Epstein-Barr Virus (EBV) EBV belongs to the gamma herpesvirus subfamily of herpesvirus. Its membrane is derived from budding of immature particles through the cell membrane which is required for infectivity. The genome is a linear dsDNA which does not normally integrate into cellular DNA but forms circular episomes which reside in the nucleus. Two epidemiological patterns are seen with EBV. In developed countries there are 2 peaks of infection, one at a very young age (1-6yrs) and the other in adolescents (14-20yrs). In developing countries infection occurs at a much earlier age (2years) by which 90% of children are seropositive. The virus is transmitted by contact with saliva, in particularly through kissing. Once infected a lifelong carrier state develops whereby a low grade infection is kept in check by the immune defences. Low grade virus replication and shedding can be demonstrated in the epithelial cells of the pharynx of all seropositive individuals. EBV is able to immortalise B-lymphocytes in vitro and in vivo. EBV is associated with several different diseases where it may act directly or as one of the several co-factors e.g. 1) Infectious mononucleosis (glandular fever) 2) Burkitt’s lymphoma 3) Nasopharyngeal carcinoma 4) Lymphoproliferative disease 5) And many others Diagnosis: Acute EBV infection is usually made by heterophil antibody test/detection of anti-EBV VCA IgM. Burkitt’s lymphoma – diagnosed by histology. The tumour can be stained with antibodes to lambda light chains which should reveal a monoclonal tumour of B-cell origin. In over 90% cases cells express IgM at cell surface. NPC – diagnosed by histology. The determination of titre anti-EBV VCA IgA screening for early lesions are used for monitoring treatment. Patients with non specific symptoms should be given a thorough examination. Vaccines against EBC prevent primary EBV infection should be able to control both BL and NPC. The vaccine would be useful in seronegative organ transplant recipients, severe IM and male proliferative syndrome carriers. The antigen chosen for vaccine development is MA antigen gp 340/220 as antibodies against this antigen are virus neutralising, this is currently being tried in Africa. Conclusion: Large group of dsDNA, ubiquitous, medically important especially in pregnancy and immunocompromised individuals.
0 Comments
Influenza is an infectious respiratory illness caused by an infection with a influenza virus. Flu has substantial incidence in children & adults <65 yrs. Not everyone gets sick when infected; the common symptoms include headache, fever, cough, sore throat, chills, fatigue and cold like symptoms. It is generally common in the infant and elderly as they are more susceptible to disease.
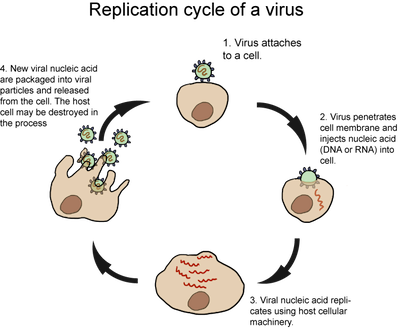
Influenza is known as orthomyxoviridae, it is an enveloped virus with a helical nucleocapsid. Influenza virus has segmented genomes, ssRNA with 8 different RNA molecules. Therefore various strains can be formed, each segment codes for one protein hence different strains of influenza. Haemagglutinin binds to cell surface receptors. Neuraminidase is an enzyme that breaks down sialic acid and is involved in protein assembly. Both H and N are involved in immunity in regards to IgA. There are three types of influenza A, B and C others are known to exist. Influenza A undergoes drift and shift. Influenza B only undergoes shift. Meaning antigenic shift is the re-assortment of gene segments from different strains and antigenic drift is the change of amino acid sequence of H and/or N from genetic mutations. Most changes occur through drift i.e. H3N2 Hong Kong flu may have been due to the change in H. Others known as H5N1, H9N2 also exist, however H1N1 and H3N2 (Sydney strain) are currently circulating. MODE OF TRANSMISSION Traditionally, influenza viruses have been thought to spread from person to person, primarily through large-particle respiratory droplet transmission (such as when an infected person coughs or sneezes near a susceptible person). Transmission via large-particle droplets requires close contact between the source and the recipient, because droplets generally travel only short distances (approximately ≤6 ft) through the air, but then settle out of the air. Indirect contact transmission via hand transfer of influenza virus from virus-contaminated surfaces or objects to mucosal surfaces of the face (nose, mouth) may also occur. Airborne transmission via small-particle aerosols in the vicinity of the infectious person may also occur; however, the relative contribution of the different modes of influenza transmission is unclear. All respiratory secretions and bodily fluids, including diarrheal stools, of patients with influenza should be considered infectious; however, the predominant source of infection is respiratory secretions. Viable influenza virus is rarely detected in blood or stool of infected patients. Most healthy adults who are ill with influenza may shed virus and be infectious to others from the day before symptom onset to 5–7 days after symptom onset; children, severely immunocompromised people, and more severely ill people, including those who are hospitalized, may shed influenza virus for ≥10 days after the onset of symptoms. The replication of influenza is similar to that of an animal viral replication. Influenza enters the host cell by haemagglutinin binding to sialic acid found on glycoprotein receptors of host cell. The cell endocytoses the virus. In the acidic environment of endosomes, virus changes shape and fuses its envelope with endosomal membrane. This is followed by a signal to release the viral nucleocapsid into host cell cytoplasm. The nucleocapsid travels to the host nucleus. Once in the host nucleus, primary transcription produces proteins for replication. It involves cap snatching where viral endonuclease (PB2) cuts 5’ methylguanosine cap as well as 10-13 nucleotides from RNA. This is used as a primer for transcription of PB1, viral transcriptase. In influenza A and B, 10 proteins result from translation of 8 segments of genome including H, N, PB1, PB2 etc... Once the initial proteins are made, the 8 + cRNA sense are made from 8 – cRNA sense. These lack 5’ capped primer as well as 3’ poly (A) tail found in mRNA. From cRNA, - RNA sense is produced. Various proteins help – RNA to exit the nucleus and enter into the cytoplasm of host cell. Meanwhile in the cytoplasm, H and N have undergone glycosylation, polymerisation and acylation. H, N and M2 travel together to the plasma membrane. There it meets with other matrix protein, M1 and begin the budding process, where the viral particle buds. N finally destroys the sialic acid receptors on the membrane thus allowing the viral particle to leave the cell. EPIDEMIOLOGY Seasonal Influenza Infection with seasonal influenza viruses is common. In temperate climates, most cases occur during the winter months. The influenza season in the Northern Hemisphere may begin as early as October and can extend until May, and the influenza season in the Southern Hemisphere may begin in April and last through September. In tropical and subtropical areas, infection with influenza virus may occur throughout the year. CDC estimates that from 1976 through 2006, annual seasonal influenza-associated deaths in the United States ranged from a low of approximately 3,000 people to a high of approximately 49,000 people; about 90% of these deaths occur among people aged ≥65 years. Influenza virus infections can cause disease in all age groups. Infection rates are highest among infants and children, while rates of severe illness (including death) are highest among people aged ≥65 and people of any age who have underlying medical conditions that place them at increased risk for complications. Children aged <2 years have rates of influenza-related hospitalization that are as high as those in the elderly. Zoonotic Influenza Influenza A viruses circulate in many different animal populations. The primary reservoirs for influenza A viruses of all subtypes are wild birds. Influenza viruses found in birds are typically referred to as avian influenza viruses. Influenza A viruses are also endemic in pigs globally and in horses in many countries. Other animal species may also become infected with influenza A viruses, including domestic poultry and marine mammals. During the 2009 pandemic, infections of domesticated cat and dogs, ferrets, turkeys, a cheetah, and other animals were also reported. Human infections with animal-origin viruses are uncommon, but they occur. Before the 2009 H1N1 pandemic, occasional swine influenza infections among humans were reported in the United States and elsewhere. In addition, more than 500 human infections with highly pathogenic avian influenza A H5N1 (HPAI-H5N1) have been reported globally since 2003. Human infections with HPAI-H5N1 are particularly concerning because of the high case-fatality ratio of approximately 60% and because this virus is widespread among poultry in some countries in Asia and the Middle East. Thus far, however, the spread of HPAI-H5N1 viruses from one ill person to another has been reported rarely and has thus far been limited, inefficient, and unsustained. Human infections with other avian influenza viruses have also included avian H7N7, H7N2, and H9N2. No sustained transmission of these other avian influenza viruses has been documented, but these viruses, along with H5N1, still have the potential to result in a pandemic. In addition, a lot of people continue to be infected by a particular subtype of influenza (H7N9) Avian influenza A. It was found in China on March 2013. It previously detected in birds in the past but was not observed in animals nor humans like in 2013. The World Health Organisation on January 2015 performed a risk assessment and have ultimately discovered that public health risk has not been altered since the initial assessment that took place on October 2014. To see the report for October 2014. Here is information below: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/riskassessment_h7n9_2Oct14.pdf?ua=1 However, since then, infections in both humans and birds have been observed. The disease is of concern because most patients have become severely ill. Most of the cases of human infection with this avian H7N9 virus have reported recent exposure to live poultry or potentially contaminated environments, especially markets where live birds have been sold. This virus does not appear to transmit easily from person to person, and sustained human-to-human transmission has not been reported. Pandemic Influenza A global pandemic was declared by the World Health Organization after 30,000 confirmed cases of 2009 pandemic influenza A (H1N1) virus had been reported from 74 countries. The virus spread from North America to the rest of the world. Severe illness resulting from infection with this virus was associated with risk factors such as chronic medical conditions, immunosuppression, pregnancy, young age, morbid obesity, and being a member of an indigenous population. However, in contrast to the epidemiology of seasonal influenza, CDC estimates that almost 90% of deaths from this virus occurred among people aged <65 years. The 2009 pandemic influenza A (H1N1) virus continues to circulate and was included as a component of the 2010–11 seasonal influenza vaccine. The H5N1 avian influenza outbreak in Hong Kong 1997 In the latter half of 1997, an outbreak occurred in Hong Kong whereby 18 persons were infected by an avian influenza A, serotype H5N1. Of these 6 died, and 3 others were severely ill. The source of the outbreak was infected chickens and the outbreak stopped after all the chickens were slaughtered in the territory. Large-scale serological studies carried out showed that workers in the poultry industry were particularly at risk of infection although none complained of any symptoms. There was evidence of limited human to human transmission. It was postulated that the strain of avian influenza involved was unusually virulent; it had multiple basic amino acids near the cleavage site of the haemagglutinin protein, which as a result may render the haemagglutinin susceptible to a wider range of proteases. Since that outbreak, no more cases have occurred. In 1999, there were reports of human infections by avian influenza A H9N2 in Hong Kong and in Mainland China. However, all these cases were very mild and it is thought that the virus was unlikely to pose a large public health risk. TREATMENT Influenza epidemics are responsible for massive disruption to industry, and for a significant number of deaths, particularly in the elderly and the very young. At present, treatment of influenza is entirely symptomatic. Salicylates should be avoided in children because of the link with Reye's syndrome. 2 compounds, amantidine and ribavirin, with antiviral activity against influenza have been identified and may be of value. Amantidine - this compound inhibit the growth of influenza viruses in cell culture and in experimental animals. Amantidine is only effective against influenza A, and some naturally occurring strains of influenza A are resistant to it. The mechanism of action of amantadine is not known. It is thought to act at the level of virus uncoating. The compound has been shown to have both therapeutic and prophylactic effects. Amantidine significantly reduced the duration of fever (51 hours as opposed to 74 hours) and illness. The compound also conferred 70% protection against influenza A when given prophylactically. Amantidine can occasionally induce mild neurological symptoms such as insomnia, loss of concentration and mental disorientation. However, these symptoms quickly developed in susceptible individuals and cease when treatment is stopped. The therapeutic and prophylactic activity of amantidine is now generally accepted and numerous analogues of this compound have been prepared. Rimantadine is not as effective as amantadine but is less toxic. Prophylaxis with 200mg of amantadine per day for 5 to 6 weeks or for the duration of the influenza A outbreak is not recommended for all persons. However, elderly persons with chronic underlying disease, institutionalized persons, staff and patients in hospital, close contacts of an index case, and patients who cannot receive influenza A vaccine due to sensitivity to egg protein may benefit from prophylaxis. Amantadine can also be used for therapy of uncomplicated influenza A infections. The recommended dose is 200mg for 5 days. Rimantadine may be used in place of amantadine for prophylaxis and the treatment of uncomplicated influenza A infections. Rimantidine - this compound is similar to amantidine but has fewer side effects. It is approved by the FDA for the treatment and prophylaxis of influenza A infection in persons one year or older. It should be used for uncomplicated influenza A infections only since it is thought to be less effective than amantidine. Amantadine and rimantadine resistant viruses are readily generated in the laboratory. Resistance has been linked to changes in the M2 protein. To date, the emergence of resistant influenza A has been documented primarily in young children undergoing therapy with rimantadine. The resistant viruses had been transmitted and caused influenza. The universal susceptibility of all types of naturally occuring influenza A isolated from man and animals suggests that resistance will be found only in individuals treated with the drug. The reason for the natural selection of the susceptible phenotype of influenza A in nature is not known. Zanamivir - the rational approach to drug design has led to the design of several potent inhibitors of influenza neuraminidase. Zanamivir was the first neuraminidase inhibitor available for clinical use and is effective against both influenza A and B. Because of its poor bioavailability, zanamivir must be administered by inhalation. Zanamivir had been shown to be effective and devoid of significant side effects in clinical trials. It is now approved by the FDA for use as treatment for influenza A and B in persons 12 years or older but not for prophylaxis. Oseltamivir - oseltamivir is another neuraminidase inhibitor but unlike zanamivir, it can be given orally. Like zanamivir, it had been shown to be effective and devoid of significant side effects in clinical trials. It is approved by the FDA for use as treatment for influenza A and B in persons 18 years or older. It is also approved for prophylaxis in persons 13 years or older. Its lack of side effects would make particularly attractive in a family setting although its higher cost compared to amantidine and rimantidine should be taken into account. Last week I wrote about what are viruses, the different types and classification system. Today I will look out how they replicate. In other words, how do they grow and proliferate. Replication consists of the following steps: Adsorption: The first stage of the virus is to attach to the cell. This is achieved by the interaction of receptors located on the cell membrane of the host cell and the virus particle. Also animal viruses only infect certain organisms and certain tissues within a host. Usually the receptors on the host cell will join to hormones or other molecules required for the cell to function properly. Most of the receptors are from a group called glycoproteins and are involved in the immune system. Animal cells membranes have micro-domains called lipid rafts that are involved in virus entry and assembly. E.g. the receptors for enveloped viruses are concentrated in lipid rafts. When the virus binds to these receptors, the host cell is tricked and the virus is endocytosed. The surface site of the virus that comes into contact with receptors of the host cell may simply consist of a caspid structure. In some viruses the binding site is at the bottom of the surface. Penetration: Once the virus has been adsorbed it can then penetrate the cell membrane and enter the host cell. Soon after the viral; nucleic acid prepares for replication. Before this can occur, some viruses have shed the caspid protein. – called Uncoating. The mechanism of penetration and Uncoating vary depending on the virus. Enveloped viruses may enter the host cell in a different way than a naked virus. Some viruses only inject their nucleic acid, while others make sure the RNA/DNA polymerase also enter the host cell. Virus penetration may occur in two modes: (1) Fusion of the viral envelope with the host cell membrane or (2) entry by endocytosis (1) The envelopes of some viruses fuse directly with the host cell plasma membrane which may involve glycoproteins that bind to the plasma membrane proteins. After attachment, a number of things happen: the membrane lipids rearrange, the adjacent halves of the contacting membranes merge and a pore is formed. The nucleocaspid enters the cell cytoplasm. (2) non-enveloped viruses and some enveloped viruses enter the cell via endocytosis. They may be engulfed by receptor mediated endocytosis to form coated vesicles. The virions attach to clathrin-coated pits; these pits pinch off to form the vesicles containing the virus. These vesicles then fuse with endosomes after the clathrin has been removed. Depending on the virus the nucleocaspid may escape before or after the fusion. Endosomal enzymes are involved in the Uncoating process of the virus and low pH also aids in this process. The viral envelope may fuse with the Endosomal membrane and the nucleocaspid may be released into the cytoplasm. Once in the cytoplasm, the viral nucleic acid may be released (the caspid may be completely uncoated or may still function while attached to the caspid. Naked viruses lack an envelope so cannot go through the fusion process. Instead the caspid has a conformational change. The altered caspid contacts the vesicle membrane which will either release the nucleic acid through a pore or the membrane may rupture. Genome replication: Replication and Transcription in DNA Viruses- the early part of synthesis is controlled by early genes, their role once the host cell has been taken over is to synthesise the viral DNA and RNA. Some animal viruses inhibit DNA, RNA and protein synthesis of the host cell. Some viruses stimulate the synthesis of macromolecules in the host cell.DNA replication usually occurs within the host cell nucleus (poxvirus –in cytoplasm). Messenger RNBA (early mRNA) is transcribed from DNA by host enzymes (poxvirus use viral polymerase). Below are illustrated the modes of replication of few viruses.
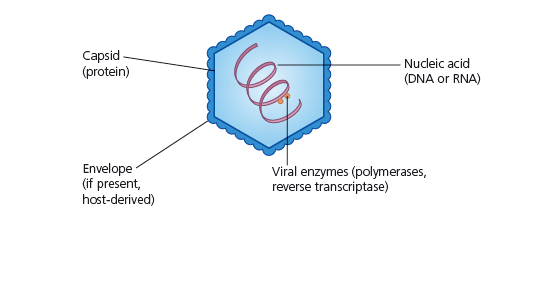
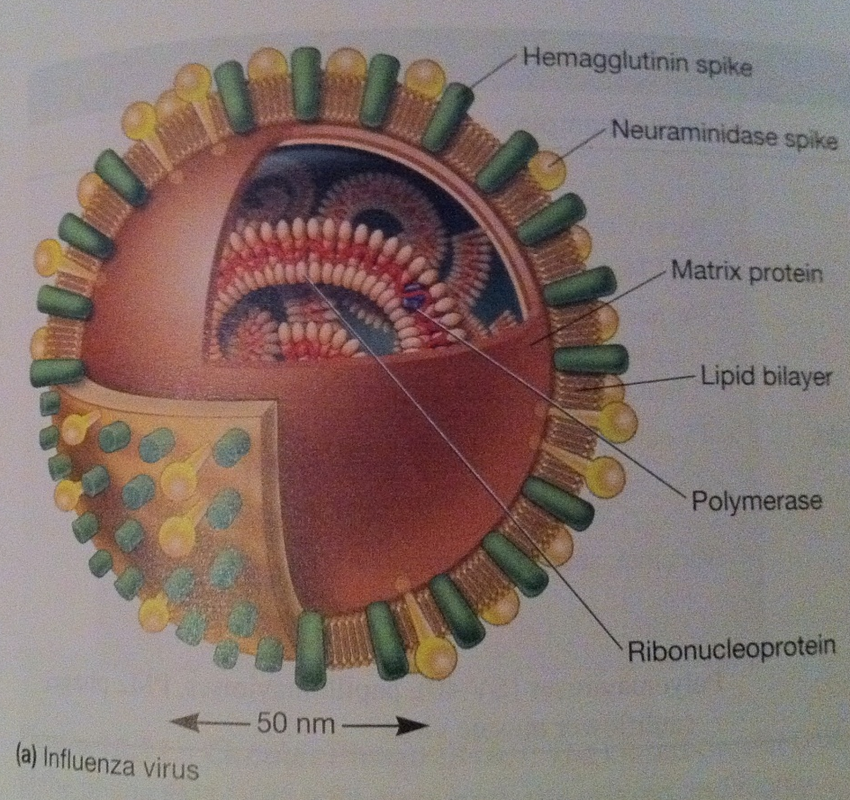
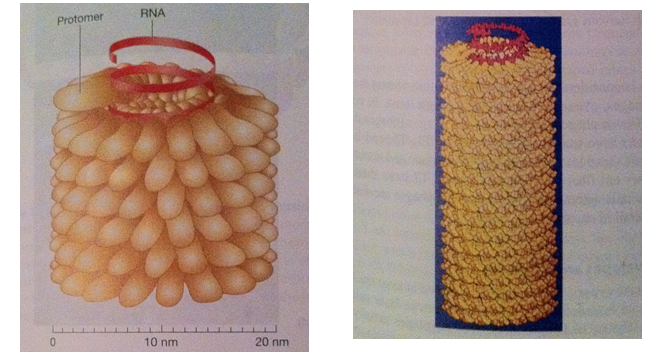
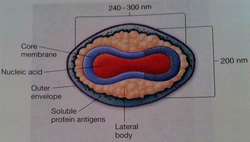
Assembly of Virus Caspid:
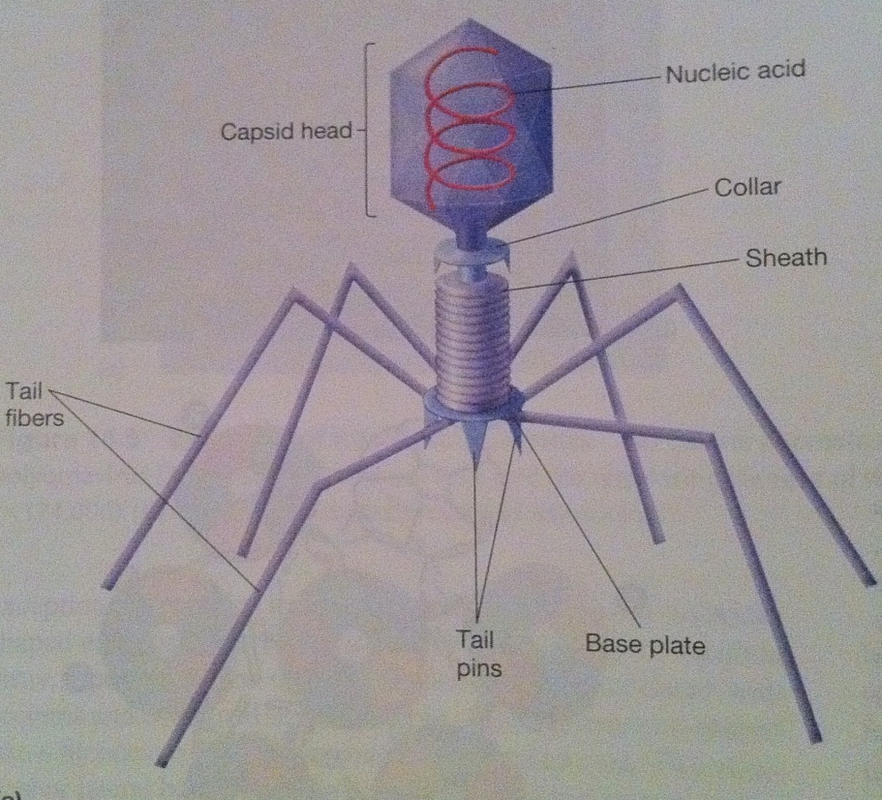
Late genes are involved in the synthesis of caspid proteins, which self-assemble to form the caspid. It is seen that during the formation of the icosahedral empty procaspids are formed and then the nucleic acid is inserted. The site of morphogenesis varies between different viruses. The assembly of the caspid for enveloped and naked viruses is similar (except for poxviruses). Large clusters of paracrystaline clusters of either complete virions or procaspids are seen at the site of virus maturation. These are assembled in the cytoplasm by complex processes which begin off by the enclosure of a portion of the cytoplasmic matrix by the construction of a new membrane. The new DNAS condenses passes through the membrane and moves to the centre of the virus. Nucleoid and elliptical body construction occur within the membrane. Virion Release: There are different ways in which enveloped and naked viruses are released. Naked viruses are usually released host cell lysis. Enveloped viruses have multiple steps before being released. Virus-encoded proteins are incorporated into the plasma membrane. Then the nucleocaspid is released and the envelope the envelope is formed via budding process. In many virus families, a matrix (M) protein is attached to the plasma membrane and aids in budding. Most envelopes arise from the plasma membrane. (In herpesvirus the budding and the envelope formation usually involve the nuclear membrane. (Golgi apparatus, ER, and other internal membranes may be used). It has also been discovered that actin filaments also aid in virion release. Many viruses change the actin microfilaments in the host cell cytoskeleton. Plan for upcoming articles in virology series 1. Influenza in more detail. 2. Herpes in more detail. 3. The relation between viruses and cancer This is part of a mini series where I will look at the following Article 1: What are viruses, the different types of viruses? Article 2: How are viruses replicated? Article 3: What is the relation between viruses and cancer. What is a virus? A sub-cellular organism, no metabolic activity outside a host cell which it requires for replication. It is an obligate, intracellular parasite. Structure of Viruses The size of viruses range from 10-400nm in diameter, there are those that can be seen through a light microscope, but most viruses can only be seen through an electron microscope. Smallpox – 200nm Poliovirus – 28nm Parvovirus – 18-22nm The largest viruses can be seen in the electron microscope transmission but cannot be seen under light microscope. They usually consist of nucleic acid (either one or more DNA or RNA molecules [not both] wrapped in a coat of protein. They depend entirely on the host cell for their metabolism and multiplication. Some viruses can have additional layers that consist of carbohydrates, lipids and additional proteins. They can exist in the following phases: extracellular and intracellular. Extracellular viruses consist of few enzymes and cannot reproduce independent of living cell. Intracellular viruses exist as replicating nucleic acids that induce host metabolism to synthesize virion particles. Viruses are the most widespread of all pathogens affecting nearly every species from animals to protozoa, bacteria and plants (well known tobacco mosaic virus). The basic structure a virus consists of following: Viral genomes: Viruses can have four possible nucleic acids: (single stranded DNA) ssDNA, (Double-stranded DNA) dsDNA, (SingleStranded RNA) ssRNA, (Double-stranded RNA)dsRNA. All four of these can be found in animal viruses, most plant viruses will have ssRNA and most bacterial viruses contain dsDNA. The size of the genetic material will also vary depending on the virus: e.g. MS2 viruses have 4000 nucleotides - code for 3/4 proteins. T-phages can have genomes of 1.0-2.0 x 105 nucleotides – synthesize 100s of proteins. Viral Envelopes Many viruses are bound by an outer membrane layer called an envelope that covers the protein caspid. Animal virus envelopes usually arise from host cell nuclear or plasma membranes (their lipids and carbohydrates are normal host constituents). These envelopes help the virus enter the host cell, and glycoprotein help to indentify and bind to receptor sites on the hosts membrane. The viral can then fuse with the host’s membrane. However the virus buds will often die or be weakened this is because the lipid bilayer envelops are sensitive to desiccation (dryness), heat and detergents. Envelope proteins are coded for by virus genes and may even be projected on the envelope surface as spikes (peplomers). These spikes are involved in helping the virus attach to the surface of the host cell. These spikes differ amongst viruses therefore can be used to identify certain viruses. The envelope is flexible membrane structure giving the virus a variable shape (pleomorphic). Some envelopes (e.g. in the bullet-shaped rabies) the envelope is attached to the underlying nucleocaspid giving the virus a constant shape. Influenza virus is an example of an enveloped virus. Spikes project about 10nm from the surface. These spikes posses the enzyme neuraminidase which helps in the release of mature virions form the host cell. Other spikes will have hemagglutinin proteins. These proteins attach the virion to red blood cells and cause them to clump together (agglutinate). Proteins that are found on the outer envelope surface are generally glycoproteins. A nonglycosylated protein, (M) or ‘matrix protein’ are found on the inner surface of the envelope and helps stabilize it. Caspid A caspid is a protein shell of the virus that consists of oligimeric structural subunits made of protein called protomers; the basic sub units of caspids are capsomers that is an outer covering of the protein that protects the genetic material of viruses. The nucleic acid encodes the genetic information of a virus that is arranged in to genomes. There are different types of genomes usually single stranded DNA (ssDNA) or double stranded DNA (dsDNA). DNA viruses usually belong to the group one or group two of the Baltimore classification system for the viruses. However in infected cells ssDNA is usually expanded to ds DNA genome in infected cells. Some viruses are naked and some contain envelopes. The spike of a virus usually helps the virus to invade the host for example the influenza virus has two types of spikes, one which is the haemagglutinin protein (HA), that fusses with the host cell, and The neuraminidase that helps the newly formed particles to bud out from the host cell membrane. What are the different types of caspids? Caspids can be present in three forms: helical, icosahedral and complex. Helical: Shaped like hollow tubes with protein walls. A virus example with this type of caspid is the tobacco mosaic virus. The protomers arrange themselves into a helical arrangement which produces a long, rigid tube, 15-18nm in diameter and 300nm long. The caspid enclose an RNA nucleic acid. The size of the helical caspid is influenced by both the protomers and the nucleic acid it encloses. Icosahedral: This is a regular polyhedron with 20 equilateral triangle faces and 12 vertices. The icosahedral is the most efficient way to enclose a space. Very few genes are required to form this type of caspid. The caspid is constructed from ring or knob-shaped units called capsomeres, each made up of 5/6 protomers. Pentamers- Have 5 sub-units. Hexamers- Have 6 sub-units. Pentamers are usually at the vertices of the icosahedron, while hexamers form the edges and triangular faces. Complex: Most viruses will have an icosahedral or helical caspid, but there are also viruses that do not fit into either category (e.g. poxviruses and bacteriophages). Poxviruses are the largest animal viruses and can be seen through a phase-contrast microscope. In the poxvirus the dsDNA is contained in a nucleoid which is shaped like a biconcave disk and surrounded by a membrane. Two elliptical or lateral bodies lye between the nucleoid and the outer envelope: a membrane and a thick layer covered by an array of tubules or fibres. Viral enzymes Most viral enzymes are located within the caspid. E.g. influenza virus: it uses RNA as its genetic material and carries an enzyme that synthesizes RNA using RNA as a template. This enzyme is called RNA-dependent RNA polymerases. Bacteriophages Bacteriophages are more complex such as T2, T4 and T6 phages have a binal symmetry structure. It is called this as the phages contain a head that resembles an icosahedron and a tail that resembles a helical. The head is elongated by one or two rows of hexamers in the middle and contains the DNA genome. The tail is made up of a collar joining it to the head, a central hollow tube, a sheath surrounding the tube, and a complex base plate. The sheath is made up from 144 copies of the gp18 protein arranged in 24 rings, each containing six copies. In T phages the base plate is hexagonal and has a pin and a jointed fibre at each corner. Not all phages will have the all the structures mentioned above (T1-lack sheath, T3 – lack tail fibres). Baltimore Classification – 7 classes 1. dsDNA Adenoviruses, Herpesviruses, Poxviruses 2. ssDNA (+) sense Parvoviruses 3. dsRNA Reoviruses 4. ssRNA(+ ) sense Picornaviruses, Togaviruses 5. ssRNA(-) sense Orthomyxoviruses, Rhabdoviruses 6. ssRNA(+)sense with DNA intermediate. Retroviruses 7. dsDNA with RNA intermediate Hepadnaviruses (e.g. hepatitis B) On sunday I will look at how Viruses are replicated.
Stay tuned! This article will summarise what the brain looks like, the purpose of its use, and what the Quran says about this particular organ, the different types of brain tumours, how is it tested, types of treatments available.
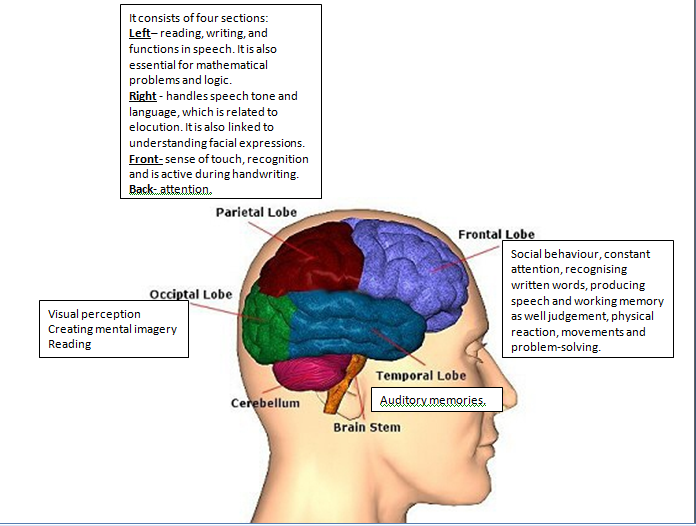
THE BRAIN IS DIVIDED INTO SECTIONS AND SUB-SECTIONS... The brain consists of three main parts the cerebrum, cerebellum and brain stem. The cerebrum consists of different regions known as lobes that have specialised functions. The cerebellum is responsible for posture, balance and the vital centres (cardiac/respiratory/vasomotor centres). The brain stem role is to focus on the vital centres as well as the motor and sensory pathways. What is the Quran and what does the Quran say about the brain? The Quran is the holy book in which Muslims believe in and are sacred words of Allah (God). It was revealed in stages to the Prophet Muhammad peace be upon him in Arabic. Previously, for many centuries, it was theoretically believed that the role of the frontal part of the brain (forehead) known as the prefrontal vortex of the cerebrum is vision because it was located near the eyes. Today, amongst the physiology books you will discover that the function of this particular area of the cerebrum is to plan and initiate movements that occur in the anterior portion of the frontal lobes and this is a region of association cortex. It is also a location for motivation where it is thought to be functional centre for ‘aggression’. In the Quran, Allah states in 96:16 ‘A lying sinful forehead’. The Quran notes that those who disbelieved will be dragged from the forehead. Thus how can today’s explanation of this particular area of the cerebrum linked to ‘lying, sinful forehead’? The prefrontal cortex is responsible for planning and initiating good and bad actions as well as for telling the truth and lies. This was revealed 1400 years ago? Isn’t this miraculous? Professor Keith Moore notes that scientists have only discovered these facts 6 decades ago. Malignant, Benign, cancer, neoplasm, tumour...what do they all mean? A tumour is a neoplasm that refers to a mass. A neoplasm is an abnormal growth of cells that grow quicker than normal cells and will continuously grow unless treated. Benign means non-cancerous and is generally harmless. Benign tumours can be easily treated however if left untreated it can grow large and lead to other diseases due to its size. Another reason why benign tumours need to be treated is it can mimic malignant tumours. Malignant tumours are cancerous and are often resistant to treatment and can spread to other areas of the body and they can sometimes come back after have been removed. What is a brain tumour? Brain tumours can be divided into primary or secondary. A primary brain tumour initiates in the brain and have not spread there from a different organ. Secondary brain tumours are those that have spread to the brain from a different organ of the body. What are the causes of brain tumour? The cause for majority of brain tumours is unknown. Tumours are not infectious so they cannot be passed onto others. Age – risk of developing brain cancer increases with age despite it can develop at any age. Gender – common in men than women Radiotherapy – People who have had radiation exposure to the head, it can increase risk of brain tumour. Genetic conditions – For instance, neurofibromatosis type 1 and type 2, tuberous sclerosis, or the following syndromes: Von Hippel-Lindau, Li-Fraumeni, Gorlin.Turcot Other possible risk factors such as viruses, mobile phones have been suggested. What are the types of cancers? Glial cells Glial cells are supporting cells of the brain. Majority of brain tumours develop from glial cells and are known as gliomas. There are different types of glial cells, thus different forms of gliomas are formed: 1) Oligodendroglial tumours – These are made from glial cells called oligodendrocytes whose role normally is to cover nerve cells (myelin sheath). 2) Astrocytic tumours – Most common type of glioma and develop from star-shaped cells called astrocytes. 3) Mixed glioma - This is where a range of different types of glial cells are involved. For instance, oligo-astrocytomes – a mixture of oligodendrocytes and astrocytes. Ependymal tumours These are rare type that develops from ependymal cells. They line the ventricles (fluid spaces in brain) and central canal (area on spinal cord). Meningioma These arise from the meninges. Meninges are membranes that cover the brain. Tumours can occur in any area of the meninges and grow very slowly. Pineal region tumours. There are two cerebral hemispheres: right and left. The pineal gland is located where the two hemispheres are linked together. It is a rare tumour and is made up of different types of cells that determine the rate of the growth where some are slow-growing and others are fast-growing. Spinal tumours This is normally caused by pressing on spinal nerves. In some cases, a tumour in the lower area of the spinal cord can cause loss of control of bladder or bowel. Medulloblastomas It is a type of primitive neuroectodermal tumour (PNET). PNET are cells remaining from early stages of development of the baby in the womb. This is amongst the common malignant brain tumours in children but is rare in adults. They develop in the cerebellum then spread to other areas of the brain. There are rare cases where medulloblastomas spread out of the brain to lymph nodes or the lungs. Haemangioblastoma. This is a rare type of slow-growing, benign tumour and develops from cells that line blood vessels. Central Nervous System (CNS) lymphoma. It is a malignant tumour that develops in the lymphatic system. Lymphatic system is part of the immune system that aims to protect the body from infection and diseases. The system consists of lymph nodes, spleen, thymus and bone marrow. In rare cases, they only affect the brain and is known as primary CNS tumours. Pituitary tumours. They are benign and are called pituitary adenomas. Acoustic neuroma They are benign tumours that develop in the acoustic/auditory nerve. This nerve controls balance and hearing and is covered by Schwann cells. This is where the tumour initiates and is known as schwannoma. They are normally found in adults especially in people with neurofibromatosis type 2 (NF2); a genetic condition. How to treat brain tumours? There are a range of treatments available such as chemotherapy, radiotherapy, surgery that can be used alone or a combination of these treatments. The type of treatment given to the patient is dependent on a number of factors: grade, type, size, position of the tumour as we as the overall general health. Surgery can range from a simple operation such as having a biopsy to a more complicated to having it completely removed. Normally for majority of primary tumours, surgery is the first treatment where the tumours can be removed without causing damage towards the rest of the brain tissue. However, there are some types of brain tumours where surgery should be last treatment used, not used immediately or not at all. For instance, some gliomas that are low-grade can be treated with radiotherapy without chemotherapy or can be closely monitored. Lymphomas can be treated with a combination of chemotherapy and radiotherapy rather than surgery. After surgery, radiotherapy is often used if the tumour has not been completely removed or abnormal cells are left behind. However for high grade tumours, radiotherapy is still recommended even if the tumour that is obvious has been fully surgically removed. The next topic I will talk about is viruses? what are viruses? what is the relationship between viruses and cancer?. This will be released on Wednesday God Willing. |
A GOOD HEALTH MAKES YOU RICHHealth is crucial in every single person’s life. Its something that money can’t buy. Thus a good health makes you rich so look after it. Archives
May 2017
Categories |

 RSS Feed
RSS Feed