The involvement of Penicillium and Pseudomonas in the cause and/or cure of human microbial disease.4/29/2015 There are many microbes which humans have contact with everyday that are harmless; however, there are other microbes that cause disease known as pathogens. Penicillium is a genus of sac fungi called Ascomycota. Its cell wall is composed of chitin and its body known as thallus, has high-branched cell filaments of hyphae (Willey, 2008:p.631). These cellular characteristics give Penicillium strength to perform its applications. Pseudomonas is a gram-negative, rod-shaped proteobacteria. They have a lipopolysaccharide outermembrane whose role is to protect the bacteria from host defences by creating a permeable barrier (Willey, 2008:p.60). The Pseudomonas genus has 60 species and is divided into rRNA homology groups based on their properties such as pathogenicity because there are many species that can be positioned under one characterized group (Willey, 2008:p.556). Both microbes, Penicillium and Pseudomonas, are involved in the cause and/or cure of human microbial disease.
The involvement of Penicillium in microbial disease is the production of β-Lactam antibiotics called penicillin. Originally, P.notatum was used to make penicillin however; there are now other species like P.chrysogenum that manufacture penicillin in vast amount. These drugs are bactericidal because they tackle disease by binding to Penicillin-binding Proteins (PBPs) such as transpeptidase that are responsible for peptidoglycan crosslinkages. This enzyme-substrate complex inhibits the bacterial cell wall, losing its function but causes less damage to the host; this is known as selective toxicity (Willey, 2008:p.837). Phenoxymethylpenicillin (Penicillin V), is effective using this mechanism against gram-positive bacteria like Streptococcus pyogenes; a bacterium that causes skin infections. However, Phenoxymethylpenicillin is not very effective against gram-negative bacteria because they have a protective outermembrane and contain β-Lactamase enzyme that inactivates penicillin by hydrolysing a bond in β-Lactam ring. This emphasises the contribution of Penicillium to treatment of disease because its species are used to manufacture penicillin which are effective against diseases caused by gram-positive bacteria. Other penicillins are effective against gram-positive and gram-negative bacteria. For instance, Ampicillin is less susceptible to inactivation by gram-negative bacteria and inhibits transpeptidase competitively because this semi-synthetic drug is a modified version of the natural penicillin by an addition of an amino group. This emphasises the involvement of Penicillium in the cure of disease because the antimicrobial activity can vary with individual penicillins. Some are narrow-spectrum drugs that kill specific bacteria, others are broad-spectrum that effect different bacteria. The involvement of Pseudomonas in disease is causing them by three stages: attaching to bacteria, invading it and causing infections. The origins of Pseudomonas’ invasive infections are mainly due to its species; P.aeruginosa. P.aeruginosa is a nosocomial, antibiotic-resistant pathogen. People with Pseudomonas infections typically have low immune systems such as cystic fibrosis where Pseudomonas produces ‘slime’ in the lungs causing pneumonia (Singleton, 1995:p.286). Another infection caused by P.aeruginosa is ‘Hot Tub Rash’ the common name given to skin infection called dermatitis. It spreads via contaminated water which indicates how this pathogen can tolerate water environments and cause infection (CDC, 2010). This illustrates Pseudomonas’ contribution to disease because P.aeruginosa causes many pathogenic infections due to its cellular characteristics and ability to form biofilms which protects it from environmental factors. Other Pseudomonas species are involved in the treatment of human microbial disease. For instance, P. flourescens extracts are used to produce an antibiotic called Mupirocin. Mupirocin is effective against gram-positive bacteria like staphylococci to treat skin infections topically. For instance, impetigo that commonly affects children who have other skin problems because it is contagious (Lancini,1995:p.178). Studies on Mupirocin have shown that it inhibits isoleucy-tRNA synthetase enzyme which can affect protein synthesis in the bacterium (Capobianco, 1989). This indicates how Pseudomonas is able to produce antibiotics against other bacteria despite itself can cause bacterial diseases. Penicillin can cause adverse side effects such as allergies where the body produces antibodies that suspect penicillin as an antigen rather than a cure. The most severe allergic reaction is anaphylaxis where bronchi are narrowed causing breathing difficulties (Singleton, 1995:p.297). Other Penicillium spp. like P.marneffei causes disseminated infection called penicilliosis in normal immune individuals. These individuals commonly have their skin, lungs and gut infected where symptoms like lesions can be mistaken for histoplasmosis that occurs in AIDs patients. In other words, the symptoms of both infections ‘mimic’ each other (University of Adelaide, 2010). This emphasises the downside of Penicillium where penicillin causes side effects and cause disease. However, P.marneffei is the only dimorphic species in the Penicillium genus. Ultimately, microbes have major importance in disease, some can be pathogenic, and others can be beneficial to produce antimicrobial drugs. Both Penicillium and Pseudomonas are involved in the cause and cure of human microbial disease. However, Penicillium predominantly contributes to treatment of bacterial diseases but depends on its side chain that determines its effectiveness. Pseudomonas predominately causes infections due to its cellular characteristics that enable it to become antibiotic-resistant. In fact, Ticarcillin, semisynthetic penicillin is effective against P.aureginosa. Today, researchers aim to develop new semi-synthetic drugs to treat disease as the number of antibiotic-resistant microbes increase to help treat human microbial disease. References Capobianco,J., Doran,C., Goldman,R. (1989) ‘Antimicrobial Agents and Chemotheraphy: Mechanism of mupirocin transport into sensitive and resistant bacteria’ American Society For Microbiology: 33 (2): 156-163. Available online: http://aac.asm.org/cgi/content/abstract/33/2/156 Centre for Disease Control and Prevention (2010) ‘Hot Tub Rash’ Pseudomonas Dermatitis/ Folliculitis’ Available online: http://www.cdc.gov/healthyswimming/derm.htm Lancini, G., Parenti, F., Gualberto Gallo, G., (1995) ‘Antibiotics: A Multidisciplinary Approach’. New York: Plenum Press. pp.178 Singleton, P. (1999) ‘Bacteria in Biology, Biotechnology and Medicine’ 5th ed. England: John Wiley & Sons Ltd pp.286, 297 University of Adelaide (2010) ‘Mycology online: Penicilliosis marneffei’ Available online: http://www.mycology.adelaide.edu.au/Mycoses/Opportunistic/Penicilliosis_marneffei/ Willey,J., Sherwood, L., Woolverton,C. (2008) ‘Prescott, Harley, and Klein’s Microbiology’ 7th ed. New York: McGraw-Hill pp.60, 556,631,837.
1 Comment
CF is caused by the abnormal functioning of the protein CF transmembrane conductance regulator (CFTR). CFTR is only expressed in a number of cells but all cells carry the gene for the protein. CFTR is mainly found in epithelial cells and in order for CFTR to be expressed a certain amount must be produced at the right time. The CFTR protein contains 1480 amino acids and is important in regulating the flow of chloride and sodium across the epithelial surface. The function of CFTR is disrupted by changes in the base sequence, these changes are called mutations. Over 1,200 mutations have been discovered for CF but the most common is deltaF508-which is a 3 base pair deletion (of amino acid phenylalanine) and account for 76% of affected chromosomes in the UK. CF is an autosomal recessive disease, so both CFTR genes are required for the individual to be diagnosed with CF, if only one is present then the individual will be a carrier.
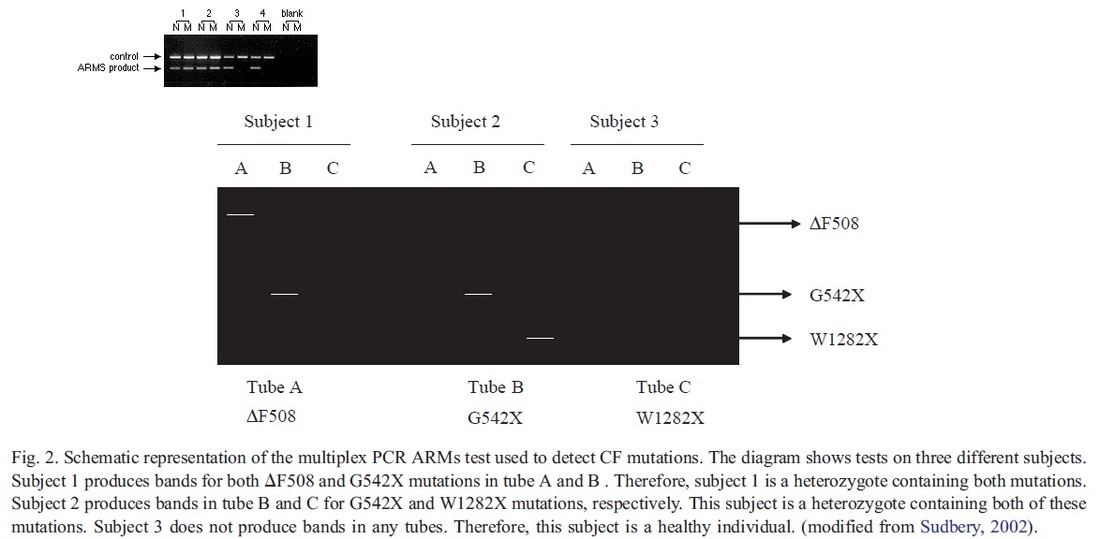
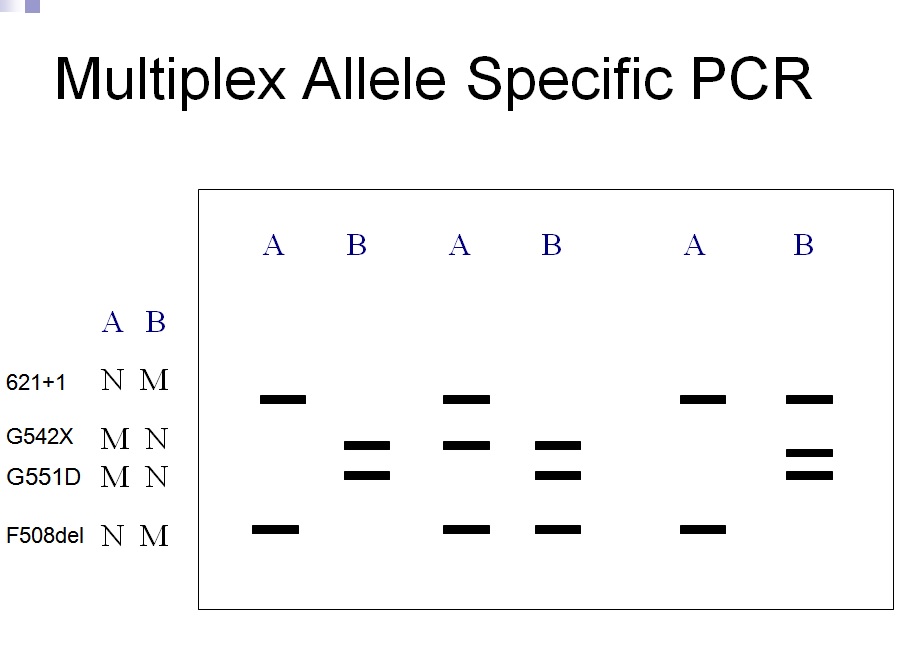
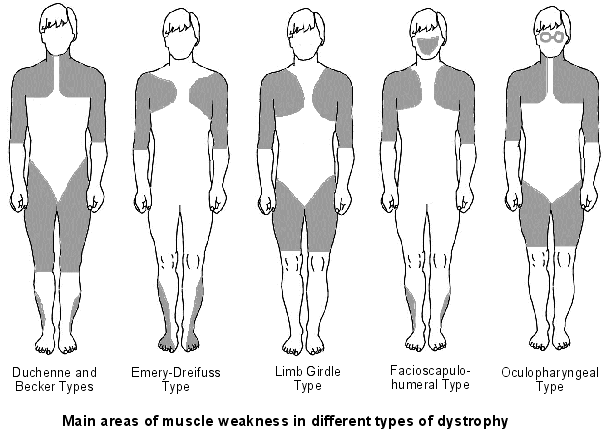
A classification system has been produced that characterises CFTR mutation depending on the effects the mutation has on the CFTR production. Class 1 are nonsense mutations as there is a premature stop codon which leads to the production of unstable mRNA or the release of incomplete proteins that are not functional. This protein is degraded before it can reach the membrane. The phenotype of patients carrying the stop mutation is severe. In Class 2 after CFTR has been translated into a peptide in the ribosome, it will go through a series of processes in the ER and Golgi apparatus. These processes are glycolisation and folding by chaperons that enable the trafficking of CFTR to the apical cell membrane. In Class 2 mutations, the CFTR protein is unable to fold correctly due to the deltaF508. The protein is retained in the ER and eventually targeted for degradation. Class 3- phosphorylation of CFTR protein by protein kinase (PK) & dephosphorylation by protein phosphatase (PP) is important in regulating CFTR chloride channel activity. Phosphorylation of the regulatory domain causes ATP to bind to the nucleotide binding domain (NBD) resulting in induction of chloride transport. In Class 3 CFTR is produced, processed and transported and inserted into the apical membrane however phosphorylation or binding of ATP does not occur. Class 4- in this class of mutation the CFTR protein is produced, processed, transported to the apical membrane. Phosphorylation and dephosphorylation also occur. In class 4, phosphorylation actually reduces chloride transport. Class 5- this class of mutations generates both abnormal and correctly spliced transcripts. These patients have a mild phenotype but with variable disease expression depending on the level of correctly spliced transcripts. Steps of diagnosing a baby with CF Neonatal screening test To diagnose a baby with CF the first test that is to be performed is the neonatal screening test. During the 1st 2 weeks of Childs life, high levels of IRT levels will be shown if CF is present. In this test a blood sample is taken from the child heel and the level of this pro-enzyme is monitored. If levels are high, then other genetic test can be carried out to find out exactly which mutations are present and detect the severity of the disease. This test is not reliable after 2 weeks of birth as several other factors may play a part in the high levels of IRT. PCR Analysis One of the 1st genetic tests that can be carried out for the diagnosis of CF is PCR analysis. This test is carried out to see if the most common mutation (deltaF508) is present which is the deletion of 3bp. PCR primers have been developed that can distinguish a normal gene from a mutant gene. With these primers a 154 bp product is produced from a normal individual and a 151 bp product is amplified from DNA of an individual with the disease. If this CF mutation is present it will show a distinct pattern from a normal pattern. If both genes have this mutation, two bands will be seen. If 1 band is present, this indicates that other the second gene has a different mutation which can be followed up by other genetic tests. Normal Carrier CF Individual Individual Individual ---------------------------------- 154 bp ___2x ___ 151 bp ___ ___2x ---------------------------------- ARMS Another test that can be performed is the amplification refractory mutation system (ARMS). This can be used to recognize the 20 most common CF mutations. In this technique, three multiplex PCR reactions are performed in parallel for each individual. Each tube contains number of separate primers capable of detecting different mutations. In each tube, sets of primer pairs are designed to anneal a particular allele to produce distinct band. A band is only produced in presence of mutant allele and the absence of the band indicates that the allele is absent; therefore positive signals are easily differentiated in order to identify the exact mutations present. Since it is possible for one of the tubes to produce no bands, there are always positive controls to show that the PCR reaction is functioning properly in each tube. The ARMS assay is accurate, rapid and easy to perform, but it does not distinguish between homozygotes and heterozygotes except for the DF508 mutation. Multiplex Allele Specific PCR Allele specific oligonucleotide (ASO) dot-blot is a widely used technique to diagnose CF. Genomic DNA from the patient is amplified by PCR and transferred onto nylon membranes as a dot-blot. Membranes are hybridized with either a radiolabelled wild type allele specific oligonucleotide (ASO) or mutated ASO. Following autoradiographic exposure, the combination of oligonucleotide hybridization is observed. Using this combination an ASO dot-blot can clearly distinguish homozygous, heterozygous and wild type subjects. The molecular basis of DMD was first determined in the 1980s. DMD symptoms usually begin to show between the ages of 3-5 and affect 1 in 3,500 patients. At the age of 20 patients are usually unable to walk so bound to a wheelchair. In 1986 Gowers observed that familial cases were more common than sporadic cases. P.E Becker discovered that there is a milder form of DMD where symptoms usually show after the age of 12 and affects 3 per 100,000 new born. He also discovered that X-linked disorders have slower progression. It does not affect reading frame but has DMD gene deletions. The protein involved in DMD is dystrophin. The dystrophin gene is the largest gene that encompasses 206 million base pairs and contains 79 exons. It is the main proteins that link the cytoskeleton to the extracellular matrix. DMD has shown variation in muscle size and penetration of connective tissue. Patients suffering from DMD have a deletion of 45 exons and in BMD 35 exons. Deletions have been found on Xp21.
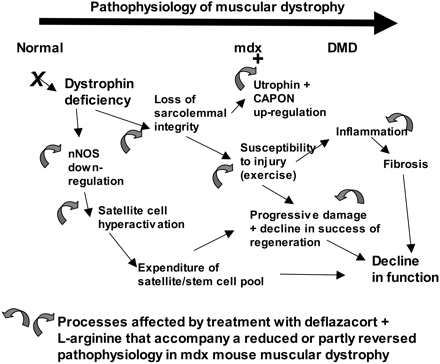
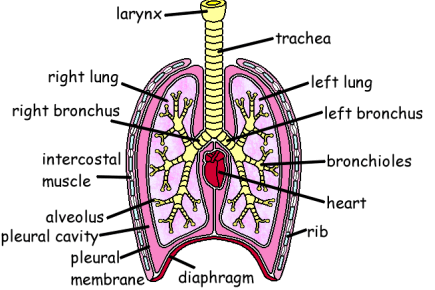
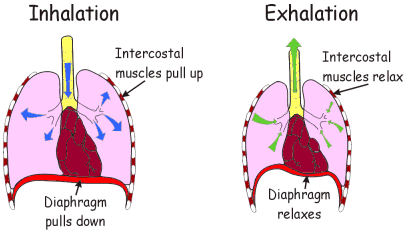
The cloning of the dystrophin gene in the 1980s led to the discovery of physiological consequences of DMD. There are three major proteins that were first discovered known as dystrophin associated protein complex (DAPC). Dystrophin is a rod shaped cytoplasmic proteins that links actin fibres in cortex of muscle cells to extracellular basal lamina (connective tissue) by forming a bridge between actin and transmembrane protein complex called (dystrophin-associated sarcoglycan complex) (DASC) located in sarcosplasma. Without dystrophin an improper complex is formed. DMD gene has 79 exons with promoter regions scatters across 2.4 Mb. The first of the proteins is known as dystroglycan. The dystroglycan complex is a membrane-spanning complex composed of two subunits, alpha- and beta-dystroglycan. Alpha-dystroglycan is a cell surface peripheral membrane protein which binds to the extracellular matrix (ECM), whereas beta-dystroglycan is an integral membrane protein which anchors alpha-dystroglycan to the cell membrane. The dystroglycan complex provides a tight link between the ECM and cell membrane. No patients have shown the mutation is the studies of Dalkillic et al (2003). The second proteins are Dystrobrevins and Syntrophins. These have been found to bind to dystrophin; these include multiple iso-forms of alpha and beta dystrobrevin and three syntrophin iso-forms. Alpha dystrobrevin is expressed in the skeletal muscle, and localized in the sarcelloma, and interacts with dystrophin. No mutations have been found but mice models with this mutation shown mild dystrophy. Three iso-forms of syntrophin have been expressed in the skeletal muscle and shown to interact with dystrophin and dystrobrevin, and bring (nNOS) nitric neurol oxide synthesis. nNOS cause vasodilation near the muscle tissue in order for blood flow to occur for contraction. Less syntrophin would mean less nNOS. No patients have shown to have this except studies of mice. Finally the last protein is the sarcoglycan-sarcospan complex. These forms of sarcoglycan interact with each other strongly that give rise to MD. DMD patients have shown to decrease in this protein and lost at the membrane. Other types of proteins that are involved are caveolac, and there is an abnormal size and number. This is found in all muscle types and interacts with C-terminal. DMD is an X-linked recessive disorder as the mutated gene is found on the X chromosome. The X chromosome is important for the development & growth. Females can be carriers of this disease if one X chromosome is affected as the other X chromosome would compensate. As for males they can either be normal with without the affected gene or affected. A female carrier can pass her affected X chromosome to son/daughter, therefore the son has a 50% of being infected and the daughter has 50% of being a carrier. There are strategies used to identify and isolate genes involved in this monogenic disorder. 1) Cytogenetic rearrangement results in DMD having location sub-chromosomal location to Xp21. Xp21 fusion with 285 ribosomal RNA nucleus on chromosome 21, allowing marker XJ1.1 à closely linked to be isolated. 2) Use of DNA from a boy called ‘BB’ who suffered from 3 x-linked disorders: DMD, chronic granulomatous disease, retinitis pigmentosa. Genes responsible for these disorders are closely linked. Appeared that BB’s X chromosome cytogentically carried a visible deletion that affected part of all 3 genes. DNA by this deletion isolated from XXXXY cell line using substractive hybridization techniques. One of these clones, pERT87 closely linked to DMD mutation sites. pERT87 and XJ1.1 clones used to map locus constructing long-range restriction map. pERT used to probe cDNA libraries resulting in isolation of cDNA clones together spanned 14-kb mRNA of locus. Structure of the Lungs The lungs are located in the chest inside a lubricated membrane called the pleural membrane. - This allows the lungs to move freely inside the pleural cavity. The lungs are connected to the outside via the trachea (windpipe). The trachea is a tube kept in a rigid shape due to rings of cartilage. The larynx or voice box is located at the top of the trachea while at the bottom end it branches into two bronchi. These lead into the lungs. The bronchi in turn branch off into smaller and smaller bronchioles. These end in tiny air sacs called alveoli. It is here that gaseous exchange takes place. The surface area of all these alveoli is very large so as to be able to absorb oxygen very quickly. The lungs are very delicate and can easily be damaged. The cells lining the airways have very tiny hair like structures called cilia on them. These cilia are coated in a sticky mucus. The beating cilia force the mucus and any particles of dirt up out of the lungs. It eventually drops down into the oesophagus so the mucus is attacked by the stomach acid, destroying any pathogens. 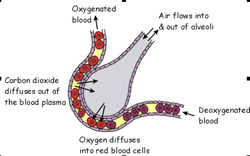 How We Breathe When we breathe, air is drawn into the lungs so that gaseous exchange can take place. The lungs are unable to draw in air on their own. The chest cavity where the lungs are positioned, is an air tight container. When we breathe in the diaphragm muscle contracts, pulling the sheet down. The intercostal muscles in between the ribs also contract which pulls the whole ribcage upwards and outwards. These together increase the volume of the chest. Air is drawn into the lungs because the the pressure inside them is lowered as the chest volume is increased. When we breathe out the diaphragm relaxes as does the intercostal muscles.This decreases the volume of the chest, increasing the pressure. This forces air out of the lungs. So it is the changing volume of the chest which causes air to enter and leave the lungs. The lungs themselves are just like balloons which are inflated and deflated. Gaseous Exchange The alveoli are the tiny air sacs at the ends of the bronchioles and the site of gaseous exchange. It is here that oxygen is absorbed into the blood while carbon dioxide is put into the air.  How We Breathe? When we breathe, air is drawn into the lungs so that gaseous exchange can take place. The lungs are unable to draw in air on their own. The chest cavity where the lungs are positioned, is an air tight container. When we breathe in the diaphragm muscle contracts, pulling the sheet down. The intercostal muscles in between the ribs also contract which pulls the whole ribcage upwards and outwards. These together increase the volume of the chest. Air is drawn into the lungs because the the pressure inside them is lowered as the chest volume is increased. When we breathe out the diaphragm relaxes as does the intercostal muscles.This decreases the volume of the chest, increasing the pressure. This forces air out of the lungs. So it is the changing volume of the chest which causes air to enter and leave the lungs. The lungs themselves are just like balloons which are inflated and deflated. Gaseous Exchange The alveoli are the tiny air sacs at the ends of the bronchioles and the site of gaseous exchange. It is here that oxygen is absorbed into the blood while carbon dioxide is put into the air.  Deoxygenated blood arrives at the alveoli in tiny blood capillaries. These have very thin walls, as does the alveoli itself. This makes it easier for the gases to pass from the air into the blood or vice versa. The deoxygenated blood has red blood cells low in oxygen and blood plasma high in carbon dioxide. The carbon dioxide diffuses from the blood plasma into the air. The oxygen diffuses from the air into the red blood cells. Blood constantly moves through the capillaries picking up O2 and giving up its CO2. Adaptations of Alveoli:
Smoking Smoking causes a number of diseases, some of them life threatening. To understand the effects of smoking you need to look at the components of cigarette smoke. Short Term Effects: Cilia can’t vibrate anymore, the air inhaled isn’t clean. Goblet cells release more mucus which makes the trachea narrower. Nicotine increases heart beat rate and blood pressure. Carbon monoxide combines with haemoglobin instead of oxygen combining with it. Carboxyhaemoglobin is formed which is stable. Less oxygen transported to cells. Diseases Caused By Tar: Chronic Bronchitis:
Lung Cancer:
Diseases Caused By Nicotine: This is the substance which makes smoking addictive. Nicotine is a stimulant which can make the heart beat faster and increase the amount of adrenaline released. It also makes the smoker more shaky. Increasing the normal heart beat rate can cause stress for the heart which can lead to heart disease. Coronary Heart Disease: Nicotine helps cholesterol deposition on walls of coronary arteries. This causes atheroma/atherosclerosis. Carbon monoxide also increases risk of blood clots forming which might results in blocking the artery. Less oxygen is delivered to cardiomyocytes (heart cells), a heart attack or failure can take place leading to death. Carbon Monoxide This is created due to incomplete burning of the tobacco. This gas binds irreversibly to the haemoglobin in red blood cells preventing them from carrying oxygen. This will make the smoker more out of breathe. If the smoker is pregnant then the amount of oxygen which is being passed on to the developing foetus is reduced. This slows down the growth of the foetus as it develops. |
A GOOD HEALTH MAKES YOU RICHHealth is crucial in every single person’s life. Its something that money can’t buy. Thus a good health makes you rich so look after it. Archives
May 2017
Categories |
 RSS Feed
RSS Feed